An Allocation Framework for Childhood Cancer
Yoram Unguru MD, MS, MA
“Unpleasant choices are intrinsic to the problem of medical lifesaving therapy selection; they are the very essence of the matter.”
–Nicholas Rescher (1969) [1]
Shortages of life-saving chemotherapeutics and supportive care agents are pervasive and enduring. These shortages represent a true public health crisis, and surprisingly, have failed to garner greater attention within the medical community or the public at-large. In the United States, shortages of drugs, including chemotherapy and supportive care agents, have become a “new normal.”
Shortages of medications and essential medical resources have a long-standing history and provide an important perspective when examining current scarcities of life-saving chemotherapy and supportive care agents. One of the first large scale drug shortages in the early 1920s involved insulin [2] and was followed in the 1940s by shortages of penicillin, which affected far greater numbers of patients [3]. In the 1960s, the medical community grappled with shortages of dialysis machines [4]. More recently, clinicians and patients have experienced shortages of seasonal influenza and H1N1 influenza vaccines. Perhaps one of the most enduring US shortages is organs for transplantation, with over 120,000 Americans in need of an organ at the time of this writing [5]. Many U.S. hospitals lack adequate health care providers, as evidenced by the dearth of skilled nurses [6], while recent natural disasters such as Hurricane Katrina [7], and infectious disease outbreaks like Ebola [8], highlight the difficulty of identifying practitioners willing to provide care in these situations.
Shortages of insulin, penicillin, and dialysis machines are especially instructive when considering the method by which each was ultimately allocated. Like many current drug scarcities, both insulin and penicillin shortages were the result of an inability to manufacture adequate supply, while scarcity of dialysis machines was the result of limited financial resources to pay for the therapy.
Dr. Frederick Banting, one of the discoverers of insulin, was responsible for its prioritization. Banting’s allocation decisions were often biased and arbitraryand were influenced by “emotional, political, and personal appeals,” such that acquaintances and the politically well-connected received priority over others [9]. Penicillin shortages occurred during the height of the Second World War. The decision to preferentially allocate penicillin to US soldiers and not civilians was made by the Committee on Chemotherapeutic and Other Agents [3]. This decision was made without stakeholder engagement and caused an outcry among the larger public who disagreed with the decision-making rationing process and with the failure to disclose the criteria for patient selection.
Decisions about allocating dialysis were determined by a 7-member panel that has become known as the “Seattle God Committee” or “God Squad” [10]. In addition to relying on medical criteria such as prognosis and health status, the committee assigned priority based on social worth; churchgoers and those with dependents (i.e., parents) received priority allocation over non-church-goers and non-parents. Each of these examples sheds light on the limitations associated with individual and committee-based allocation of life-saving medications and emphasizes the importance of a transparent and public prioritization process.
The Problem
Over the past 10+ years, drug shortages have become increasingly commonplace. Chemotherapy (and supportive care) agents are particularly prone to scarcity and are consistently ranked among the top 5 drug classes in short supply. Like many of the drugs that are in short supply, most affected chemotherapy agents belong to the class of older, generic, sterile “injectables.” In fact, since 2001, between 50%-75% of all drugs on the US “short list” are sterile injectables. As most chemotherapy is administered via injection, oncology practices are disproportionately affected by the shortage. Injectables largely comprise the backbone of proven and standard life-saving regimens for children and adults and children with cancer are particularly vulnerable, as there exist few, if any alternative agents.

Figure 1. Reasons for Drug Shortages as Determined by UUDIS* During Investigation (2015). Herman & Walter Samuelson Children’s Hospital at Sinai, Johns Hopkins Berman Institute of Bioethics. *Courtesy Erin Fox, Director, University of Utah Drug Information Service.
The reasons for drug shortages are complex and are especially common in the USA (see Figure 1) [11–16]; economies of scale, limited profit margins, quality failures, consolidation in the marketplace, a lack of manufacturing redundancy, inadequate market competition to drive down prices, regulatory considerations, and the federal government’s inability to negotiate drug prices all contribute to shortages.
Compelling research examining the economic drivers and business decisions that contribute to US drug shortages raises serious concerns. The generic chemotherapy market has become consolidated with merging of suppliers and outsourcing of drug components. As drug manufacturers often contract other companies to make their drugs, it is not publicly known who makes what for whom so there is no way to truly know what the supply chain is and no publicly available data on which manufacturers farm-out drug production. As recently demonstrated, having fewer chemotherapy drug suppliers is associated with a higher likelihood of shortages. Moreover, the strongest risk factor for a shortage is the age of the drug, with older drugs significantly more likely to experience shortages [16]. This is particularly concerning as the majority of chemotherapy agents used to treat, and to cure, most childhood cancers are older drugs. A case in point, childhood acute lymphoblastic leukemia or ALL is the most common childhood cancer accounting for nearly one-quarter of all children diagnosed with cancer with a survival rate approximating 90%. The overwhelming majority of drugs used to treat and cure ALL have been in use for more than 50 years and over the past decade, 8 out of 10 common drugs used in the treatment of ALL, have temporarily been unavailable.
As recently reported, availability of generic drugs and chemotherapeutics in particular, is directly linked to decisions by manufacturers that either delay or prevent these drugs from becoming accessible. Such decisions are associated with considerable cost absorbed by patients, governments, and insurance companies that results in increased revenues for brand-name drug companies, prompting at least one influential group to conclude that the dual mission of helping patients while simultaneously profiting has been replaced by “a mission to make profits at any cost.” [17]
Scope of the Problem
According to data from the American Society of Health Systems Pharmacists, which monitors US drug shortages [18], drug shortages reached an all-time high in the fall of 2014, with 320 shortages. In 2015, there were 142 new drug shortages with 185 active drug shortages in the final quarter of 2015. In other words, although there were fewer new drugs in short supply, because of an inability to resolve existing shortages, the total number of active shortages remained high.
With regard to chemotherapeutics, at the end of 2015, 13 chemotherapy agents were in short supply, fewer than previous quarters and less than the record 33 chemotherapy agents that were in short supply at the end of 2013. Patients receiving chemotherapy typically require more than a single chemotherapeutic. Being able to administer a chemotherapy agent that was previously scarce does little if other chemotherapy agents used as part of the same (curative) regimen are lacking. Similarly, without the critical supportive care agents, patients with cancer rely upon, e.g., leucovorin, corticosteroids, anti-emetics, and intravenous fluid solutions, all of which have been, or currently are in scarcity, administering chemotherapy is unsafe, ill-advised, unpleasant, and even impossible.
Although fewer chemotherapy shortages is certainly “good news,” drug shortages are not isolated strictly to chemotherapeutics or to a particular drug class. Shortages occur across drug classes and have far reaching consequences. With shortages of central nervous system drugs, antibiotics, electrolytes and minerals, critical care and cardiac drugs, and even normal saline (salt water), shortages impact most patients and physicians.
Consequences
The consequences of drug shortages are far-reaching. The annual costs associated with managing drug shortages has been estimated to be $416 million [19]. Beyond the economic costs associated with drug shortages, drug shortages directly impact patients’ lives and this is especially true for children with cancer. Drug shortages in general and shortages of CASCA specifically, result in increased medication errors, delayed administration of life-saving therapy, inferior outcomes, and patient deaths [20–25] .
In 2009, the chemotherapeutic, mechlorethamine, approved for use in the US in 1949 and included as part of multiagent chemotherapy regimens for the treatment of Hodgkin lymphoma for over 50 years became unavailable due to a shortage. At the time of the mechlorethamine shortage, evidenced suggested that another chemotherapeutic – cyclophosphamide – could be safely substituted for mechlorethamine to treat certain pediatric patients with Hodgkin lymphoma. However, as reported by Metzger and colleagues [22], the 2-year event-free survival for 40 patients who received the alternative regimen with cyclophosphamide, was 12.5% lower than patients who had received the standard of care treatment including mechlorethamine (See Figure 2). The authors note that while there were no deaths at the time of their analysis, patients who received cyclophosphamide received additional rounds of toxic chemotherapy, including stem cell transplantation, the long-term consequences of which remain unknown.
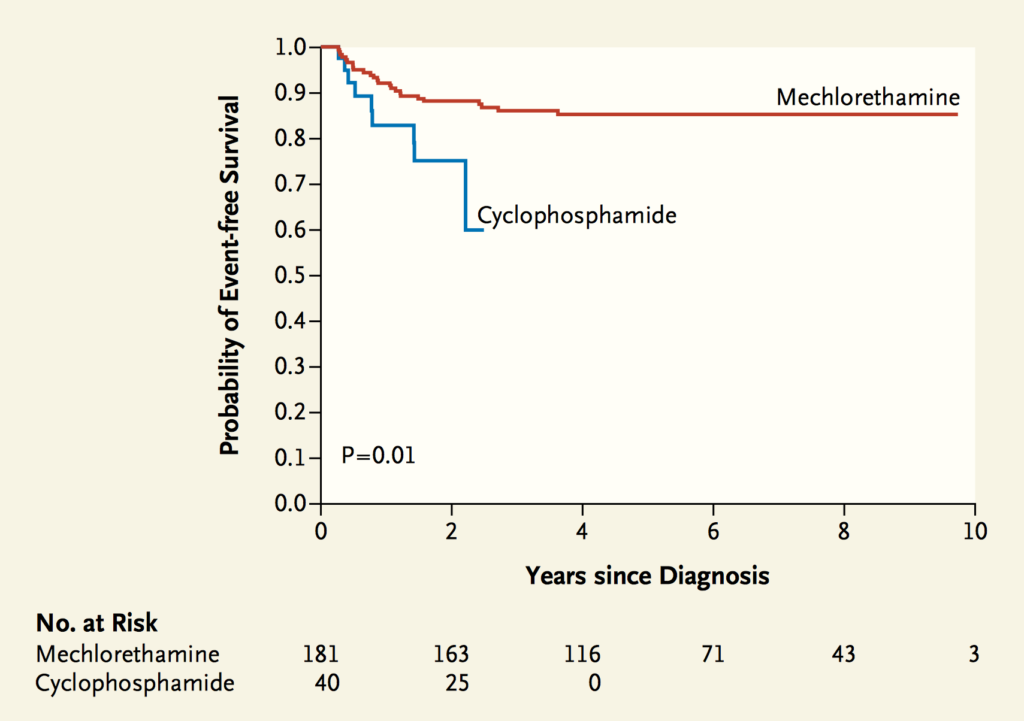
Figure 2. Event-free survival (EFS): original Stanford V regimen with Mechlorethamine compared with modified Stanford V Regimen with Cyclophosphamide for Hodgkin’s Lymphoma patients. Two-year EFS was 75% among patients who received cyclophosphamide (SE, 12.5%) and 88% among those who received mechlorethamine (SE, 2.5%; P = 0.01). *Courtesy New England Journal of Medicine, 2012. See Note 22.
A recent survey of principal investigators (PI) and pharmacists at Children’s Oncology Group (COG) affiliated centers assessed the impact of chemotherapy shortages over a 2-year period on clinical trials (both COG and non-COG trials) and patient care. Fifty percent of COG PIs reported that at least one patient they cared for who was enrolled on a clinical trial was affected by the shortages and 66% reported that at least 1 patient’s clinical care was affected by the shortages. Equally troubling, 34% of pharmacists reported at least 1 near miss or actual medication error due to the shortages [25]. Shortages of chemotherapy however, are not isolated to children. With over 1.6 million US adults diagnosed with cancer each year (compared to fewer than 15,000 children), older patients are especially at-risk.
Two surveys of medical oncologists assessed the impact of the chemotherapy shortages and lend insight to the severity of the problem. Nearly 83% of oncologists reported they were unable to prescribe their preferred chemotherapy agent; more than 75% had to make a major change in treatment such as choosing a different treatment regimen or substitute different drugs during treatment; over 40% were forced to delay the start of treatment; and 28% reported using a less-effective alternative because of a shortage [24,26]. Perhaps most concerning was the fact that nearly 70% of oncologists reported that their hospital or practice lacked any type of formal guidance for how to make decisions about allocating drugs, prompting the authors of the study to appropriately call for formal guidance in this regard.
Ultimately, drug shortages prevent clinicians from providing a reasonable standard of care and they hinder critical clinical research that is essential to guarantee ongoing advances in understanding disease processes and improving outcomes.
Ethics questions
Drug shortages present a host of ethical challenges for patients, providers, and healthcare systems. Should providers delay treatment, administer a lower dose of a medication, or skip a dose altogether? Should patients be prioritized, and if so, is the bedside treating physician or an independent neutral panel best suited to make these decisions? Should children receive priority over adults? Is it appropriate to allocate a scarce medication according to patient size, weight or developmental status? Should patients enrolled on a clinical trial receive preferential access to a scarce drug over those who opt not to participate in these trials? If allocation is deemed appropriate, what prioritization model should be relied upon? If scarce drugs are available outside approved and legal channels, through the so-called gray market, should hospitals, providers, and patients access them knowing that this practice may exacerbate shortages and that the pedigree of the drugs obtained via the gray market is not guaranteed?
To Prioritize or Not to Prioritize
Solving the drug shortage problem will ultimately require a concerted, coordinated, and cooperative effort with broad stakeholder engagement including members of government, industry, professional organizations, and patient advocates. Until such a time comes, as a society, we must be prepared to make difficult decisions about allocating scarce life-saving chemotherapy among equally deserving children.
Although prioritization of scarce health care resources is viewed by many outside the US as acceptable, necessary, and even ethical, prioritization has yet to gain wide spread support among large portions of the American public. Opponents have been quick to equate prioritization with “rationing,” and in some circles, prioritization has been framed as, “death panels.” [27] Yet rationing occurs frequently in the American health care system. Insurance companies ration when deciding which medications to include on their formularies, transplantation organizations ration when deciding how to allocate organs, and hospitals and other clinical groups assign priority access to vaccines. Moreover, a broad group of American scholars and policy experts, favor allocation as both necessary and morally permissible [28].
Irrespective of one’s position, the unrelenting shortages of life-saving medications accompanied by providers’ pleas for guidance mandates an ethical approach towards allocation of critical and essential medications. Yet, being forced to ration life-saving chemotherapeutics and supportive care agents raises serious ethical concerns. How then should providers proceed? Bedside rationing is problematic and ill suited for such a decision-making process. Bedside rationing is prone to subjective preferences and arbitrary decision-making and fails to treat similar people similarly and in so doing, violates the ethical principle of justice [29]. Other approaches towards allocation include such models as, first-come first-serve, chance-based allocation (i.e., lottery), and prioritization based on medical urgency or social value criteria according to the patient’s perceived value to society. Although allocation decisions can certainly be made conforming to anyone of these approaches, each has strengths and weaknesses and on its own is insufficient. While clinicians may combine various features of each when choosing to allocate a scarce resource, this may prove difficult in practice. Ultimately, none of these frameworks readily provides concrete guidance clinicians desire when faced with making difficult prioritization decisions among equally deserving children with cancer. What is required is an overarching framework that can be equally and fairly applied to all patients.
Allocation Process
Although ethical allocation of life-saving chemotherapy (and other medications) should include the clinician’s input, actual decision-making must not occur at the bedside and is better suited to be performed by a diverse independent panel. Given the problematic nature of bedside allocation decision-making, prioritization decisions should be made by a multidisciplinary institutional Drug Shortage Committee or similarly appointed body with appropriate stakeholder representation (e.g., Pharmacy and Therapeutics Committee, Ethics Committee).
To address this issue, a 7-member interdisciplinary and multi-institutional Allocation Task Force (TF) with expertise in pediatric oncology, bioethics, nursing, patient advocacy, psychiatry, research ethics, palliative care, pharmacy, and pharmacology was convened to create an ethical and defensible allocation framework.
Functioning as a hypothetical drug shortage committee, the TF reached consensus on an ethical framework, which delineates a process for actual rationing of life-saving chemotherapy and supportive care agents. In establishing its framework, the TF considered the various existing allocation schema and emphasized a consistent approach grounded upon ethical, legal, and socio-cultural considerations. Our guidance [30] represents a systematic recommendation aimed to minimize bias as might occur when individual clinicians or institutions are forced to make bedside rationing and prioritization decisions.
Ideally, decisions about allocating a particular drug in a given circumstance should be supported by evidence-based recommendations, yet such guidance to choose one patient over another rarely exists. Our framework provides an approach to these challenging situations. Importantly, it will serve to alleviate some of the tension individual clinicians may feel when confronted with having to make bedside decisions that can be inefficient, uncomfortable, prone to subjective preferences, and (understandably) reliant upon clinicians’ primary obligation of beneficence.
An independent panel of peer-consultants with expertise in pediatric oncology, law, regulatory affairs, pharmacology, bioethics, and advocacy reviewed a preliminary version of the report. The consultants’ feedback was incorporated into a revised report. The final report, endorsed by the leadership of the Children’s Oncology Group (COG) and the American Society of Pediatric Hematology/Oncology, provides reasoning for explicit decision-making in the face of an actual drug shortage and specifically aims to assist COG member-institutions navigate this difficult decision-making process.
The Framework
Faced with a drug shortage, the TF recommends a 2-step process. Step 1 includes strategies to mitigate an existing shortage based upon maximizing efficiency and minimizing waste. Step 2 elucidates actual prioritization (across and within diseases) grounded upon a modified utilitarian model that maximizes benefit according to total lives saved / life-years saved. Select examples of mitigation strategies include the following:
- Drug shortages should be viewed as a public health crisis. Not only will this result in greater public awareness, it also provides a mechanism for potential remediation.
- Hospitals should not order more drug than they typically require in a given period, i.e., hospitals should not hoard drugs.
- Drug manufacturers and distributors may serve as “gatekeepers,” internally allocating drugs by verifying a hospital’s ordering history.
- Hospitals that lack a drug required by a patient should attempt to secure a supply for that patient from another institution or refer the patient to another institution able to provide the necessary care.
- Patients receiving similar therapies should be brought to the hospital/clinic on the same day to share vials that are otherwise meant for single-use.
- If the preferred drug brand or strength is not available, contact manufacturers directly as alternate product sizes (i.e., larger or smaller vial size) may be available.
- If appropriate, an alternate dosing approach should be administered (e.g., lower or less frequent dose).
- If scarcity is expected to be short-lived, the scarce drug should be administered out of sequence.
- If feasible, compound drug on own or acquire from a commercial compounding pharmacy.
- When US drug manufacturers are unable to address a shortage in an expedient and timely manner, FDA should investigate the feasibility of securing an adequate supply of the drug from a non-US supplier until the shortage has been alleviated.
- If stability and sterility profile supports doing so, consider extending drug usage beyond typical and accepted practice (i.e., administer an expired drug).
Faced with prioritization, allocation decisions must be reasoned, explicit, transparent and public. Such accountability is more likely to gain the public’s trust that institutions are acting in a legitimate and fair manner and that limit-setting is based upon values and principles (recall the unfavorable public responses surrounding allocation of penicillin during WWII and subsequently dialysis).
In formulating its recommendations, the TF considered the following overarching ideals, including, but not limited to, justice, fairness, maximizing benefit, and minimizing harms. The ultimate decision-making process employed by the TF is based upon a modified utilitarian model that maximizes total benefit, emphasizing lives saved or life-years saved.
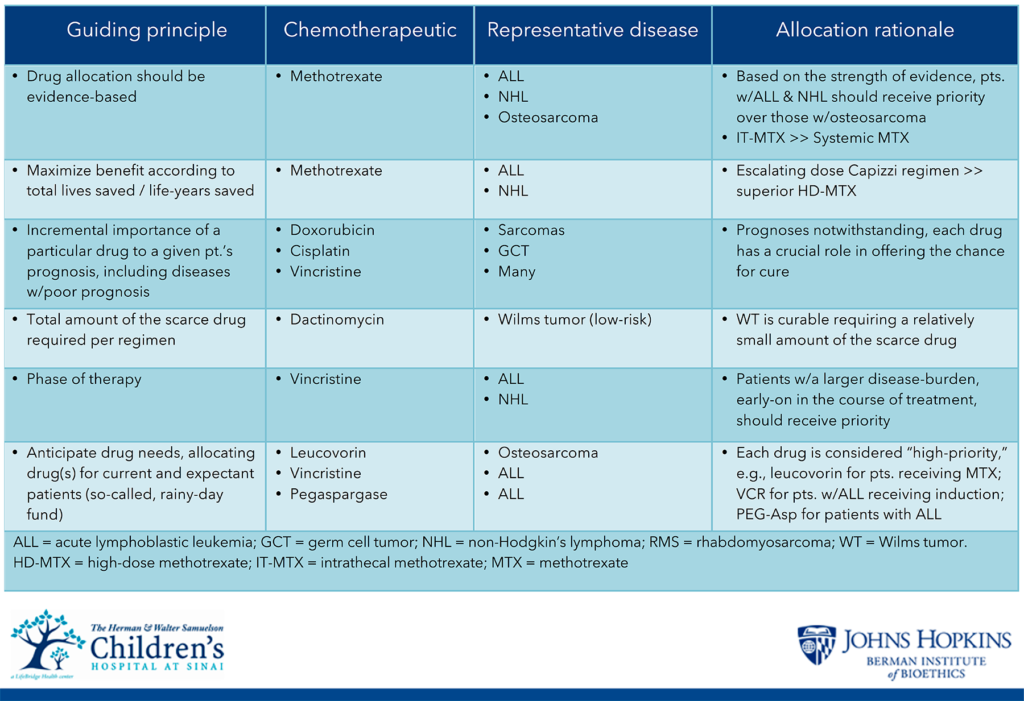
On its own however, a strictly utilitarian allocation approach is insufficient. Although saving more lives inherently is preferred to saving fewer lives, perhaps the most obvious limitation to this practice is its lack of specificity. To account for such constraints, in its deliberations, the TF recommended prioritizing chemotherapy and supportive care agents between different diseases only when the scarce drug significantly contributes to survival difference as in the case of methotrexate for treatment of ALL compared to osteosarcoma. Within a given disease state, and perhaps even a subset of disease, the TF recommends that prioritization decisions should not be based upon factors such as patient age, size, or participation in a clinical trial. Select examples of specific considerations for allocation include:
- Allocation decisions should not to be based upon age, sex, development, socioeconomic status, immigration status, race, ethnicity
- Consider “curability” and/or prognosis, including the threshold of curability.
- Allocation decisions should be based on strength of data.
- Consider a drug’s importance or need to a given patient’s prognosis.
- Consider the critical role that certain drugs have in the management of cancers with poorer outcomes.
- Consider the total amount of the scarce drug required.
- Consider the phase of therapy.
- No prioritization for clinical trial participants.
- Patients and families affected by drug shortages must be engaged and appropriately apprised of decisions about their treatments.
Conclusion
Solving the drug shortage problem is an ethical obligation and a practical problem. While healthcare rationing is inevitable, rationing life-saving chemotherapeutics and supportive agents raises many ethical challenges. Physicians, and oncologists in particular, lack sound guidance in making ethically appropriate decisions for allocating scarce drugs.
In the absence of a much-needed national advisory statement on how best to allocate scarce drugs, and until policymakers and stakeholders can prevent future shortages, physicians must be able to make thoughtful and appropriate decisions when prioritizing life-saving drugs among equally deserving patients.
The proposed recommendations provide a transparent & defensible framework to assist providers and administrators. Furthermore, it provides reasoning for explicit decision-making in the face of an actual drug shortage and aims to minimize bias as might occur when individual clinicians or institutions are forced to make difficult, and at times tragic, rationing and prioritization decisions for children with cancer.
Yoram Unguru, MD, MS, MA
Division of Pediatric Hematology / Oncology The Herman and Walter Samuelson Children’s Hospital at Sinai
2401 West Belvedere Avenue
Baltimore, MD 21215-5271
Tel: 410-601-5864
Fax: 410-601-9750
E-mail: [email protected]
The Author has disclosed no conflicts of interests
Endnotes
- Rescher N. The allocation of exotic medical lifesaving therapy. Ethics 1969;79:173-186.
- McGough LJ, Reynolds SJ, Quinn TC, Zenilman JM. Which patients first? Setting priorities for antiretroviral therapy where resources are limited. Am J Public Health 2005;95:1173-1180.
- Adams DP. Wartime bureaucracy and penicillin allocation: the Committee on Chemotherapeutic and Other Agents 1942-44. J Hist Med Allied Sci 1989;44(2):196-217.
- Levine C. The Seattle ‘God Committee:’ A Cautionary Tale. Health Affairs Blog November 30. 2009. http://healthaffairs.org/blog/2009/11/30/the-seattle-god-committee-a-cautionary-tale/. Accessed February 29, 2016.
- U.S. Department of Health and Human Services. Organ Procurement and Transplantation Network, HRSA. https://optn.transplant.hrsa.gov/. Accessed February 29, 2016.
- Juraschek SP, Zhang X, Ranganthan VK, Lin VW. United States Registered Nurse Workforce Report Card and Shortage forecast. American Journal of Medical Quality 2012;27(3):241-249. https://www.ncbi.nlm.nih.gov/pubmed/22102163. Accessed February 29, 2016.
- Fink S. Five Days at Memorial: Life and Death in a Storm-Ravaged Hospital. New York, NY: Crown Publishing; 2013.
- Sugarman J, Kass N, Rushton C, Hughes M, Kirsch TD. Translating Professional Obligations to Care for Patients with Ebola Virus Disease Into Practice in Nonepidemic Settings. Disaster Medicine and Public Health Preparedness 2015;9(05):527-530. https://www.cambridge.org/core/journals/disaster-medicine-and-public-health-preparedness/issue/821C9737613D535ECF84C5B30F4E00E3. Accessed February 29, 2016.
- McGough LJ, Reynolds SJ, Quinn TC, Zenilman JM. Which patients first? Setting priorities for antiretroviral therapy where resources are limited. Am J Public Health 2005; 95:1173-1180. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1449336/. Accessed February 29, 2016.
- Jonsen AR. The God Squad and the origins of transplantation ethics and policy. J Law Med Ethics. 2007;35(2):238-240. http://onlinelibrary.wiley.com/doi/10.1111/j.1748-720X.2007.00131.x/abstract. Accessed February 29, 2016.
- ASPE Issue Brief. Economic analysis of the causes of drug shortages. http://aspe.hhs.gov/sp/reports/2011/DrugShortages/ib.shtml. Accessed February 8, 2016.
- Dill S, Ahn M. Overview of U.S. drug shortages. November 6, 2012. www.fda.gov/downloads/Drugs/UCM327108.pdf. Accessed February 8, 2016.
- Woodcock J, Wosinska J. Economic and technological drivers of generic sterile injectable drug shortages. Clin Pharmacol Ther. 2013 Feb;93(2):170-6. https://www.ncbi.nlm.nih.gov/pubmed/23337525. Accessed February 8, 2016.
- FAQ About Drug Shortages. http://www.fda.gov/Drugs/DrugSafety/DrugShortages/ucm050796.htm#q10. Accessed February 8, 2016.
- Lipworth W, Kerridge I. “Why drug shortages are an ethical issue.” AMJ 2013;6(11):556-559. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3858608/. Accessed February 8, 2016.
- Parsons HM, Schmidt S, Karnad AB, Liang Y, et al. Association between the number of suppliers for critical antineoplastics and drug shortages: Implications for future drug shortages and treatment. J Oncol Pract. Published online before print. February 2, 2016: DOI 10.1200/JOP.2015.007237. https://www.ncbi.nlm.nih.gov/pubmed/26837565. February 2, 2016.
- Jones GH, Carrier MA, Silver RT, Kantarijan H. Strategies that delay or prevent the timely availability of affordable generic drugs in the United States. Blood 2016; Jan 27. pii: blood-2015-11-680058. https://www.ncbi.nlm.nih.gov/pubmed/26837565. February 2, 2016.
- Drug Shortages Statistics. http://www.ashp.org/DocLibrary/Policy/DrugShortages/OPA-National-Drug-Shortages.pdf. Accessed March 7, 2016.
- Kaakeh R, Sweet BV, Reilly C, Bush C, et al. Impact of drug shortages on U.S. health systems. Am J Health Syst Pharm. 2011;68(19):1811-1819. https://www.ncbi.nlm.nih.gov/pubmed/21930639 Accessed March 7, 2016.
- Institute for Safe Medication Practices. Drug shortages: national survey reveals high level of frustration, low level of safety. ISMP Medication Safety Alert! Acute Care. September 23, 2010;15(19):1-6. https://www.ismp.org/newsletters/acutecare/articles/20100923.asp. Accessed March 7, 2016.
- Institute for Safe Medication Practices. A shortage of everything except errors: harm associated with drug shortages. ISMP Medication Safety Alert! Acute Care. 2012;17(8):1-4. https://www.ismp.org/newsletters/acutecare/showarticle.aspx?id=20. Accessed March 7, 2016.
- Metzger ML, Billett A, Link MP. The impact of drug shortages on children with cancer – the example of mechlorethamine. NEJM 2012;367(26):2461-2463. http://www.nejm.org/doi/full/10.1056/NEJMp1212468#t=article. Accessed March 7, 2016.
- McBride A, Holle LM, Westendorf C, Sidebottom M, Griffith N, et al. National survey on the effect of oncology drug shortages on cancer care. Am J Health Syst Pharm 2013;70(7):609-617. https://www.ncbi.nlm.nih.gov/pubmed/23515514. Accessed March 7, 2016.
- Gogineni K, Shuman KL, Emanuel E. Survey of oncologists about shortages of cancer drugs. NEJM 2013;369:2463-2464. http://www.nejm.org/doi/full/10.1056/NEJMc1307379. Accessed March 7, 2016.
- Salazar EG, Bernhardt BM, Li Y, Aplenc R, Adamson P. The impact of chemotherapy shortages on COG and local clinical trials. PBC 2015;62(6):940-944. http://pubmedcentralcanada.ca/pmcc/articles/PMC4670038/. Accessed March 7, 2016.
- Kehl KL, Gray SW, Kim B, Kahn KL, Haggstrom D, et al. Oncologists’ experiences with drug shortages. Journal of Oncology Practice 2015;11(2):154-e162. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4371121/. Accessed March 7, 2016.
- Sarah Palin (August 7, 2009). “Statement on the Current Health Care Debate”. Facebook. https://www.facebook.com/notes/sarah-palin/statement-on-the-current-health-care-debate/113851103434/ Accessed March 7, 2016.
- Arras JD. The right to health care. In: Arras JD, Fenton E, Kukla R, eds. The Routledge companion to bioethics. New York, NY: Taylor and Francis; 2015: 3-15. https://www.routledge.com/The-Routledge-Companion-to-Bioethics/Arras-Fenton-Kukla/p/book/9780415896665. Accessed March 7, 2016.
- Sulmasy D, Moy B. Debating the oncologist’s role in defining the value of cancer care: our duty is to our patients. J Clin Oncol 2014;32(36):4039-41. https://www.ncbi.nlm.nih.gov/pubmed/25366686. Accessed March 7, 2016.
- Unguru Y, Fernandez CV, Bernhardt B, Berg S, Pyke-Grimm K, Woodman C, Joffe S. An Ethical Framework for Allocating Scarce Life-saving Chemotherapy and Supportive Care Drugs for Childhood Cancer. J Natl Cancer Inst 2016;108(6). pii: djv392. doi: 10.1093/jnci/djv392. https://www.ncbi.nlm.nih.gov/pubmed/26825103. Accessed March 7, 2016.